Vertical Integration: The Key Strategy to Bionime’s Competitive Advantage


Biochemical
Technology

R&D
Design
Manufacturing
Process

Quality
Inspection
System
Integration

Data
Integration

Global
Marketing

Customer
Management
Bionime’s self-manufacturing rate exceeds 99%, covering all aspects from biochemical technology R&D, product mechanical design, mold development, to the design of internal electronic control components, PCB processes, and the development of fully automated production and assembly machines. Through multiple testing stages such as SOC linear automatic verification, AOI optical inspection, and CCD detection, Bionime ensures the production of high-quality products.
By leveraging IoT technology, Bionime achieves production data monitoring and automatic glucose data transmission. The integration of multiple systems helps build a cross-domain glucose management ecosystem. With local production and global marketing, Bionime utilizes its CRM system to enhance customer management and service.
The Core R&D Strength Driving Innovation

Bionime develops and manufactures all its products in-house, spanning over 80 technical fields. Currently, the company holds over 400 patents across various countries. With a robust intellectual property management system in place, Bionime implements internal IP policies, encourages IP proposals, and externally works to prevent infringement of others’ rights while safeguarding its own intellectual assets.
Test Strips
The patented direct transmission design ensures the accuracy of blood glucose data. The patented embedded gold electrode offers high biocompatibility, flexibility, and conductivity, enhancing the precision of blood glucose testing.

IoT Technology
The patented smart healthcare management system connects with IoT and healthcare institution information systems, enabling more effective and real-time blood glucose data management.
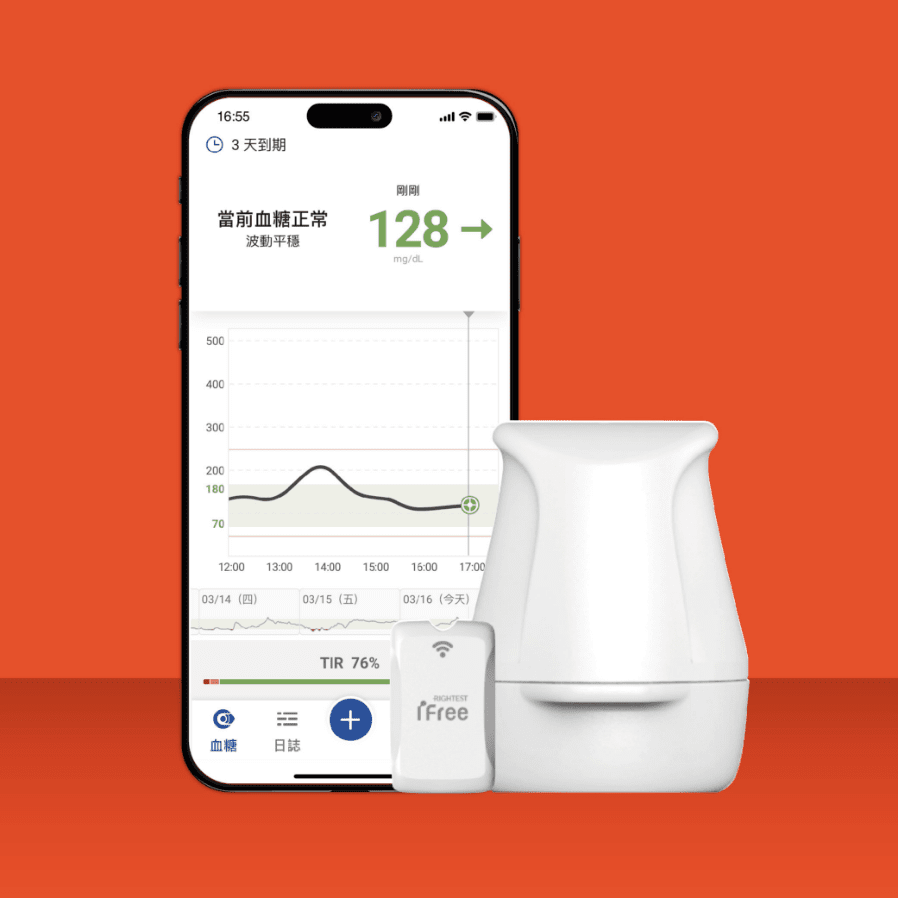
CGM
Over 400 patents have been filed, with more than 200 patents granted in key countries. The patent portfolio covers various technical fields, including electronics, mechanics, biotechnology, and software systems.
A biotech company
at the forefront of industrial automation.
Bionime, with its unwavering pursuit of quality excellence, has independently developed products and automated production equipment since its establishment. The spirit of 6 Sigma DNA has been incorporated into the development of automated equipment, and in 2019, the company was recognized by Business Today CommonWealth Magazine as the only biotech company with an Industrial 3.0 level or higher.
Bionime pursues excellence
Smart Manufacturing
Superior Quality Production

The CGM product involves over 200 production processes, with the core sensor undergoing more than 100 biochemical procedures. It is produced through fully automated machines, significantly reducing human error and ensuring the quality of every product before shipment.
Bionime has independently developed 139 types of automation machines, along with 126 AOI inspection machines for real-time monitoring. All production data and inspection results are integrated into the CIM (Computer Integrated Manufacturing) / MES (Manufacturing Execution System) software, which allows for real-time monitoring and adjustments of the automated machines. This mechanism optimizes production stability and yield, creating a low-cost, high-quality Continuous Glucose Monitoring (CGM) system and significantly enhancing market competitiveness.

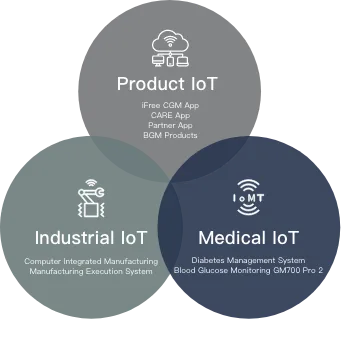
Connecting Blood Glucose
Management System
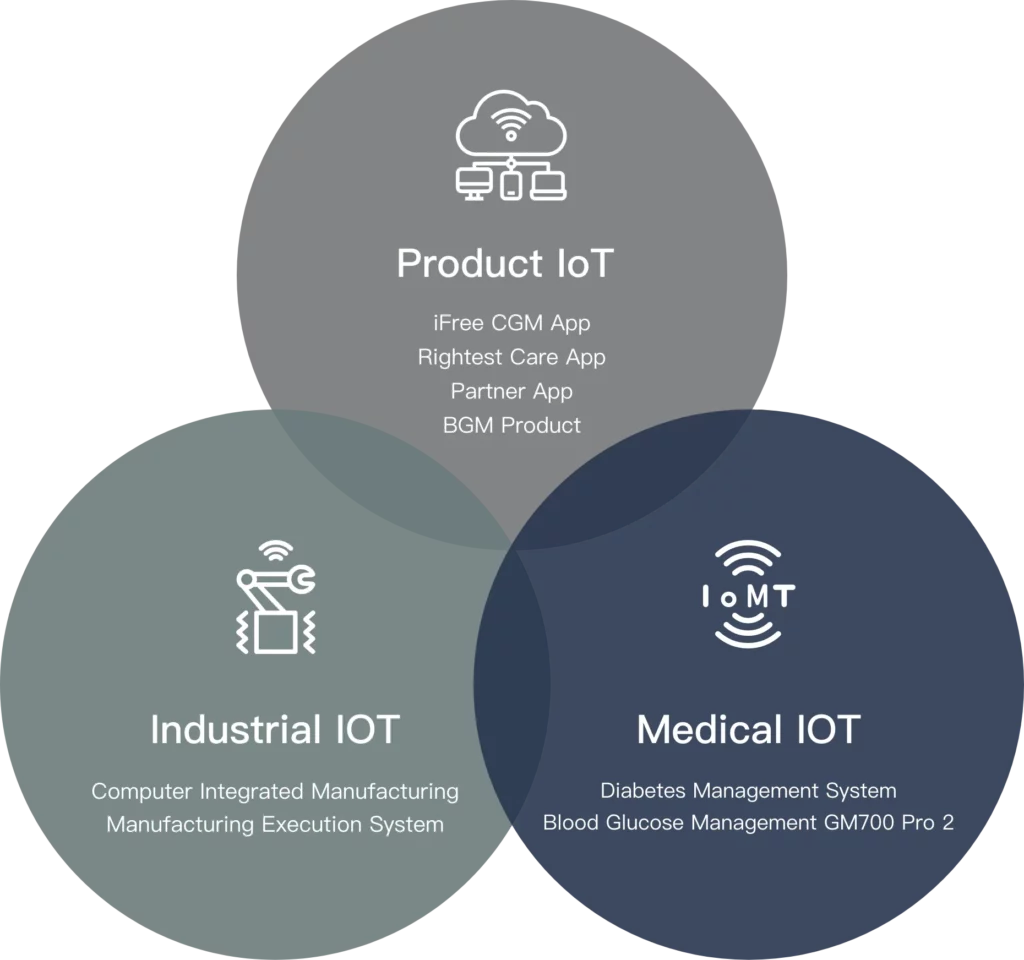
Comprehensive IOT

The integration of the blood glucose management
system with comprehensive Internet of Things
(IoT) applications not only connects the
manufacturing industry, products,
and healthcare sectors but also
brings a new solution to blood
glucose management. It
enhances users’ quality
of life while creating more
possibilities for healthcare
management.
Global Marketing, Local Customer Service

Bionime provides real-time customer support along with wide range of marketing materials.
We understand that success in the highly competitive global market comes from meticulous service and close collaboration with our customers.
Choose Bionime, and you’ll gain access to the global market while receiving dedicated local support.

Global Marketing
Strategy
Bionime participates in over 10 global medical device and diabetes-related exhibitions every year, including events like EASD, ATTD, and ADA. These exhibitions not only provide an important platform for exchanging ideas with international peers but also allow us to stay updated on the latest research findings and technological advancements.

Local Customer Service and Remote Assistance
Bionime provides local distributors with real-time customer support and a comprehensive product database through a unified global customer relationship management system, helping them offer better customer service to consumers.

Sales Resource
Support
We are committed to providing comprehensive product education and technical support, including multilingual audio-visual and print marketing materials. Our multilingual marketing materials cover a variety of languages, designed to support marketing campaigns, effectively communicate with target markets, and enhance your business performance.
Quality and Information Security Certification

ISO 27001:2022
Information Security
Standard Certification

ISO/IEC 27701:2019
Privacy Information
Management System Certification

ISO 45001:2018
Occupational Health and Safety
Management System Certification
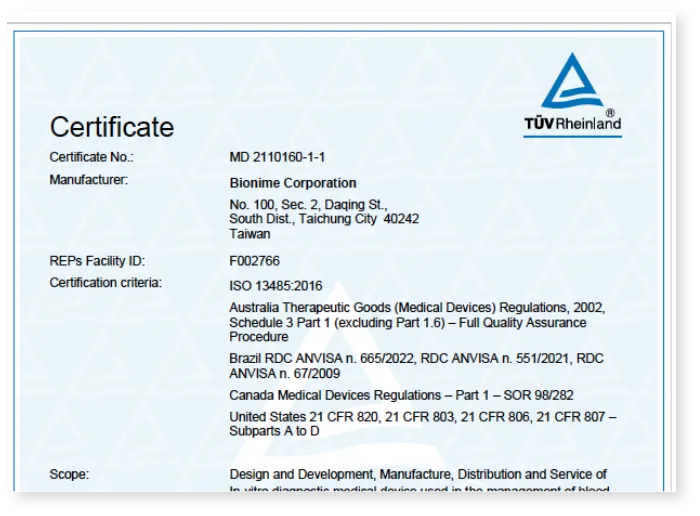
MDSAP
Medical Device Single Audit Program

QMS
Quality Management System (QMS) for Medical Devices

ISO/IEC 27017:2015
Cloud Services Information Security Management

ISO/IEC 27018:2019
Protection of Personal Data in Public Cloud Services

ISO 14064-1
Greenhouse Gas Management System Certification

ISO 13485: 2016
Medical devices — Quality management systems



Bionime Corporation │ Customer Service Hotline 0800-371-688 │ Service Hours: Monday to Friday 8:30-22:00

Vertical Integration
The Key Strategy to Bionime’s Competitive Advantage


Biochemical
Technology

R&D
Design

Manufacturing
Process

Quality
Inspection

System
Integration

Data
Integration

Global
Marketing

Customer
Management
Bionime’s self-manufacturing rate exceeds 99%, covering all aspects from biochemical technology R&D, product mechanical design, mold development, to the design of internal electronic control components, PCB processes, and the development of fully automated production and assembly machines. Through multiple testing stages such as SOC linear automatic verification, AOI optical inspection, and CCD detection, Bionime ensures the production of high-quality products.
By leveraging IoT technology, Bionime achieves production data monitoring and automatic glucose data transmission. The integration of multiple systems helps build a cross-domain glucose management ecosystem. With local production and global marketing, Bionime utilizes its CRM system to enhance customer management and service.
The Core R&D Strength Driving Innovation

Bionime develops and manufactures all its products in-house, spanning over 80 technical fields. Currently, the company holds over 400 patents across various countries. With a robust intellectual property management system in place, Bionime implements internal IP policies, encourages IP proposals, and externally works to prevent infringement of others’ rights while safeguarding its own intellectual assets.

Test Strips
The patented direct transmission design ensures the accuracy of blood glucose data. The patented embedded gold electrode offers high biocompatibility, flexibility, and conductivity, enhancing the precision of blood glucose testing.

IoT Technology
The patented smart healthcare management system connects with IoT and healthcare institution information systems, enabling more effective and real-time blood glucose data management.
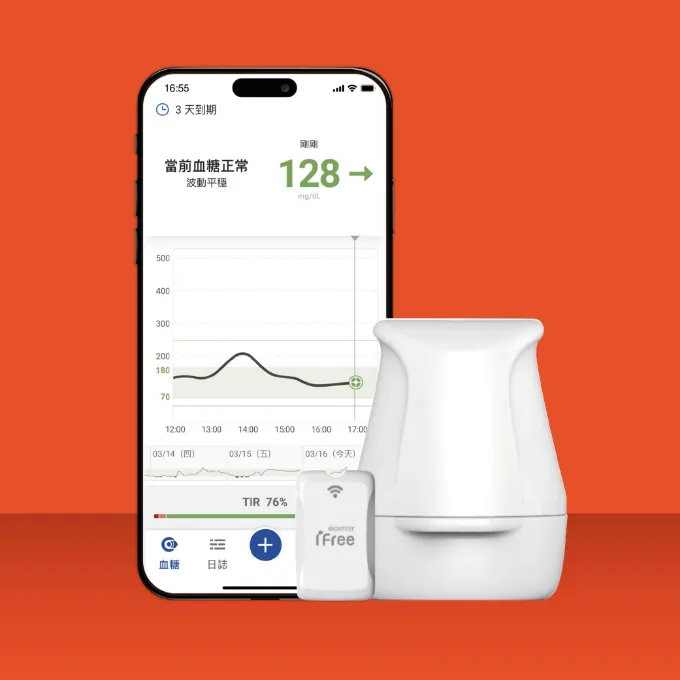
CGM
Over 400 patents have been filed, with more than 200 patents granted in key countries. The patent portfolio covers various technical fields, including electronics, mechanics, biotechnology, and software systems.
A biotech company
at the forefront of industrial automation.
Bionime, with its unwavering pursuit of quality excellence, has independently developed products and automated production equipment since its establishment. The spirit of 6 Sigma DNA has been incorporated into the development of automated equipment, and in 2019, the company was recognized by Business Today CommonWealth Magazine as the only biotech company with an Industrial 3.0 level or higher.
Bionime pursues excellence
Smart Manufacturing
Superior Quality Production

The CGM product involves over 200 production processes, with the core sensor undergoing more than 100 biochemical procedures. It is produced through fully automated machines, significantly reducing human error and ensuring the quality of every product before shipment.
Bionime has independently developed 139 types of automation machines, along with 126 AOI inspection machines for real-time monitoring. All production data and inspection results are integrated into the CIM (Computer Integrated Manufacturing) / MES (Manufacturing Execution System) software, which allows for real-time monitoring and adjustments of the automated machines. This mechanism optimizes production stability and yield, creating a low-cost, high-quality Continuous Glucose Monitoring (CGM) system and significantly enhancing market competitiveness.

Connecting Blood Glucose
Management System
Comprehensive IOT

The integration of the blood glucose management system with comprehensive Internet of Things
(IoT) applications not only connects the manufacturing industry, products, and healthcare sectors but also brings a new solution to blood
glucose management. It enhances users’ quality of life while creating more possibilities for healthcare
management.
Global Presence, Local Service Excellence

Bionime provides real-time customer support along with wide range of marketing materials. We understand that success in the highly competitive global market comes from meticulous service and close collaboration with our customers. Choose Bionime, and you’ll gain access to the global market while receiving dedicated local support.

Global Marketing Strategy
Bionime participates in over 10 global medical device and diabetes-related exhibitions every year, including events like EASD, ATTD, and ADA. These exhibitions not only provide an important platform for exchanging ideas with international peers but also allow us to stay updated on the latest research findings and technological advancements.

Local Customer Service and Remote Assistance
Bionime provides local distributors with real-time customer support and a comprehensive product database through a unified global customer relationship management system, helping them offer better customer service to consumers.

Sales Resource
Support
We are committed to providing comprehensive product education and technical support, including multilingual audio-visual and print marketing materials. Our multilingual marketing materials cover a variety of languages, designed to support marketing campaigns, effectively communicate with target markets, and enhance your business performance.
Quality and Information Security Certification

ISO 27001:2022
Information Security
Standard Certification

ISO/IEC 27701:2019
Privacy Information
Management System Certification

ISO 45001:2018
Occupational Health and Safety
Management System Certification

MDSAP
Medical Device Single Audit Program

QMS
Quality Management System (QMS) for Medical Devices

ISO/IEC 27017:2015
Cloud Services Information Security Management

ISO/IEC 27018:2019
Protection of Personal Data in Public Cloud Services

ISO 14064-1
Greenhouse Gas Management System Certification

ISO 13485: 2016
Medical devices — Quality management systems