Company Profile
Company Name
Bionime Corporation
Abbreviation
Bionime
Stock Code
4737
Market Type
Business
Industry Type
Biotechnology and Medical Care
Business
Manufacturing
Selling Medical Instrument
Biotechnology Service
Examine In Pharmaceuticals
Selling Precision Instruments
Date of Establishment
2003/4/14
Date of Listing
2010/12/23
Date of Listing
NT$ 610,145,940
Chairman
Roy Huang
Principal Office
No. 100, Sec. 2 Daqing St., South Dist., Taichung City 40242, Taiwan (R.O.C.)
Stock Transfer Agent
Chinatrust Commercial Bank
Certified Public Accountant
KPMG
Tel
886 4 2369 2388
Fax
886 4 2261 7586
info@bionime.com
Website
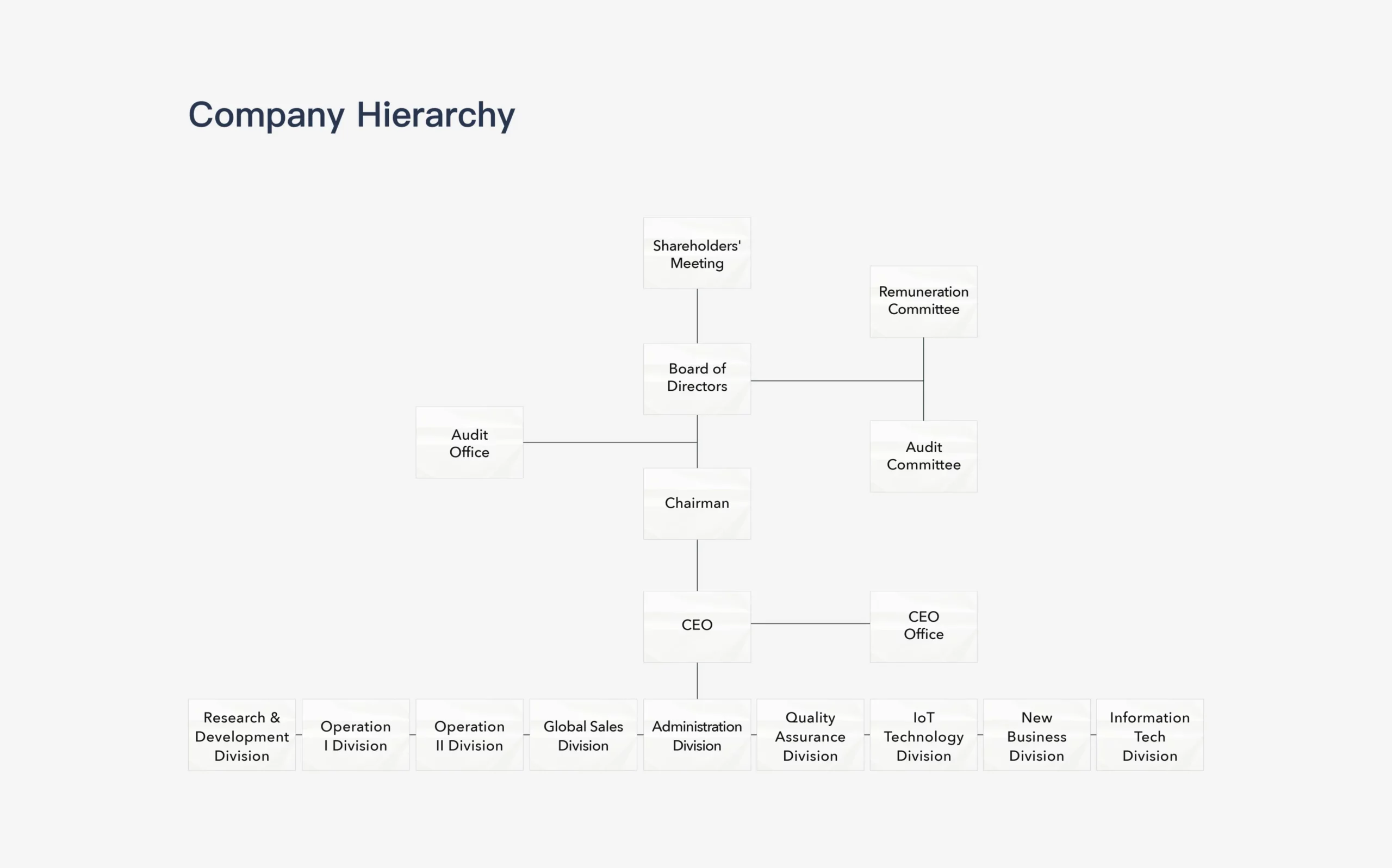
Division Functions
Division
Functions
Remuneration Committee
Assist the board of directors to implement and evaluate the company’s overall remuneration and welfare policies, and the remuneration of directors, supervisors, and managers.
Audit Committee
The Audit Committee assists the board in fulfilling its oversight of the quality and integrity of the accounting, auditing, reporting, and financial control practices of the company.
Audit Office
Report to the Board and Administration Division to take in the extent to which the operational effectiveness and efficiency objectives have been achieved as well as the reliability of the financial statements and relevant laws and regulations to comply with the relevant internal controls. Point out internal control deficiencies and any suggestions as well as abnormal matters tracking improvement.
CEO Office
Carry out all company affairs in accordance with the resolutions of the shareholders’ meeting and the Board of Directors, as well as the instructions of the Board.
Research & Development Division
Responsible for the biochemical development of blood glucose test strips, new products, clinical certification, and matters related to intellectual property and patents.
Operation Division
Oversee the company’s production, outsourcing, logistics management, mold development, and automation matters to achieve the company’s annual production plans and goals.
Sales Division
Oversee the company’s product sales, customer service, market development planning, new product development plans, IoT product service planning and design, as well as product and brand marketing communication planning and execution.
Administration Division
Oversee the company’s financial management, accounting, human resources, and administrative affairs.
Quality Assurance Division
Responsible for the company’s quality system, quality monitoring and improvement, and the validation of new product development.
IOT Technology Division
Responsible for the software research and development, operation and maintenance of the IoT cloud platform, POCT system for remote care, as well as platform system integration and technical support.
New Business Division
Responsible for the electromechanical development and integration of products, as well as the research and evaluation of innovative product development.
Information Tech Division
Responsible for the overall information systems of the company, as well as the planning, execution, and maintenance of information integration and automation for smart manufacturing.
Internal Audit

Our company’s Audit Department directly reports to the Board of Directors and is headed by an audit supervisor. The audit unit carries out regular audits according to the annual internal audit plan and conducts ad-hoc project audits as needed. The audit department also guides each department in performing self-assessment of the internal control system and reports the results of these self-assessments, along with the execution of audits, to the relevant regulatory authorities within the specified timeframe.
Stakeholders

Supplier Area
To sustainably promote the development of Bionime’s business, it is essential to establish partnerships with suppliers across the supply chain. All partners are expected to comply with the social responsibility standards of the supply chain. Our company conducts EHS (Environmental, Health, and Safety) advocacy and audits with key suppliers, focusing on the implementation of health checks for employees, prevention of occupational hazards, fire safety, environmental hygiene, and other related areas. Suppliers are expected to fully cooperate with these requirements and make necessary improvements to ensure the fulfillment of corporate social responsibility.
Key Issues



Communication Channels, Response Methods, and Communication Frequency
Established a Secure Supply Chain Partner Management Policy, with regular two-way communication with suppliers.
Supplier Contact Point
Logistics Management Department
Manager:Joanne Chang
E-mail:joanne.chang@bionime.com
Consumer Area
Bionime Corporation, established in 2003, is currently focused on developing “Self-Blood Glucose Monitoring Systems” tailored for diabetes patients and professional healthcare institutions. By successfully integrating cutting-edge technologies in healthcare, chemistry, electronics, and precision manufacturing, Bionime has developed unique patented technologies that deliver highly accurate and stable blood glucose testing systems.Today, Bionime products are sold in over 80 countries worldwide. Our products have excelled in clinical testing and evaluations by internationally recognized third-party organizations. The unique and superior qualities of our technologies have also been featured in many prestigious international professional journals. Thanks to the outstanding performance of our products and the strong support from customers, Bionime has become a well-known brand in several countries. We are confident in our ability to become the leading brand in home blood glucose monitoring systems.
Key Issues


Communication Channels, Response Methods, and Communication Frequency
Dedicated 0800 hotline for immediate customer inquiries.
Supplier Contact Point
Customer Service Department
Assistant Manager:Zoe Ho
E-mail:info@bionime.com
Toll-Free Hotline:0800-371-688
Employee Area
Bionime offers multiple channels for feedback and has established an employee mailbox, allowing staff to express their opinions freely. Additionally, company-wide operational updates are communicated through email and the internal corporate website.
Key Issues




Communication Channels, Response Methods, and Communication Frequency
Regularly announcing various employee benefits (such as self-improvement activities, travel, etc.), welfare committee information, performance assessments, two-way feedback sessions, training course details, employee suggestion boxes, and diverse communication channels like ‘Meet the CEO’ sessions for new hires.
Supplier Contact Point
Human Resources Department
Manager:Fish Chen
E-mail:hr@ bionime.com
Investor Relations Area
Our company values building strong communication channels with investors. If investors have any questions, they can contact our designated representative.
Key Issues
Company financial statements, business-related, and operational information.
Communication Channels, Response Methods, and Communication Frequency
1. Irregularly announcing company operational information on the Public Information
Observatory and Bionime’s official website.
2. Holding an annual shareholders’ meeting to update shareholders on the company’s operational status.
3. Hosting an annual institutional investor conference to brief institutional investors on the company’s operations.
4. Establishing a spokesperson system for periodic communication with investors.
Investor Contact Window
Acting Spokesperson
Manager:Sherry Huang
E-mail:investor@bionime.com
Board of Directors
The Board of Directors prioritizes the interests of the company and its shareholders. Its members are diverse, with professional backgrounds and industry experience, working together to ensure effective corporate governance.
Director
Roy Huang
Key Experience
・
・
・
Chairman and CEO of Bionime Corporation
General Manager of ECHOWELL ELECTRONIC CO., LTD.
National Taipei Institute of Technology
Multiple Positions Held
・
・
・
・
・
・
・
・
・
Chairman and CEO of Bionime Corporation
Bionime Inc. Legal Chairman
Legal Chairman of Bionime Biotechnology (Shenzhen) Co., Ltd.
Legal Chairman of Bionime Biotechnology (Pingtan) Co., Ltd.
Bionime GmbH Legal Chairman
Bionime USA Corp. Legal Chairman
Bionime Malaysia SDN BHD Director
Bionime Australia Pty Ltd Director
Legal Representative and Director of BONRAYBIO CO., LTD.
Director
LENG, CHUN-SHENG
Key Experience
・
・
Chairman, CEO, and Vice President of Tonghua Dongbao Pharmaceutical Co., Ltd.
Ph.D. in Cell Biology from Liaoning Normal University
Multiple Positions Held
・
・
・
Chairman and CEO of Tonghua Dongbao Pharmaceutical Co., Ltd.
Chairman of Beijing Dongbao Biotechnology Co., Ltd.
Executive Director of Tonghua Tongbo Biopharmaceutical Co., Ltd.
Director
ZHANG, WEN-HAI
Key Experience
・
・
Vice President of Tonghua Dongbao Pharmaceutical Co., Ltd.
TCM Pharmacy Program, Harbin University of Commerce
Multiple Positions Held
・
・
・
Director of Tonghua Dongbao Pharmaceutical Co., Ltd.
Vice General Manager of Tonghua Dongbao Pharmaceutical Co., Ltd.
Chairman of Shanghai Juyi Network Technology Co., Ltd.
Director
LIOU, XIU-MEI
Key Experience
・
・
Vice General Manager of HUA ENG WIRE AND CABLE CO., LTD.
Master of Accounting, Long Island University, USA
Multiple Positions Held
・
・
・
・
・
・
・
Legal Representative and Director of Bionime Corporation
Legal Representative and Director of Xiamen Ecotek PRC CO., LTD.
Legal Representative and Director of Wafer Works Corporation
Legal Representative and Director of Asia Pacific Telecom Co., Ltd.
Legal Representative and Director of CO-TECH DEVELOPMENT CORP.
Supervisor of HUA HO ENGINEERING CO., LTD.
Manager of HUA ENG WIRE AND CABLE CO., LTD.
Director
Yanben Investment
Co., Ltd.
Key Experience
・
・
Legal Director of Advantech Co., Ltd.
Legal Representative and Director of Chuanian IoT Investment Co., Ltd.
Independent Director
ZENG, HUI-JIN
Key Experience
・
・
・
Partner, Deputy Managing Director, Chief Operating Officer of the Audit Department, and Chief Strategy Officer of PwC Taiwan
National Taiwan University/Fudan University School of Management Overseas Master’s Degree
Master of Accounting, National Chengchi University
Multiple Positions Held
・
・
・
・
・
・
・
・
・
・
・
・
・
・
・
・
・
・
Advisory Member of the Bio Taiwan Committee.
Advisor of the Biomedical Translation Research Center.
T-E Pharma Holding (Cayman) Director
Onward Therapeutics SA (Switzerland) Independent Director
HanchorBio Inc. (Cayman) Director
Legal Representative and Director of Bonraybio Corporation
Legal Representative and Director of AP Biosciences
Director of BRIM Biotechnology, Inc.
Director of New Taipei City Contemporary Legend Cultural and Arts Foundation
Director of the Chi Po-lin Foundation.
Supervisor of the Medical and Pharmaceutical Industry Technology and Development Center.
Supervisor of the Food Industry Research and Development Institute
Vice Chairman of the A Community Of Minds Pushing Forward Advancements In Precision Medicine.
Executive Supervisor of the Taiwan Bio Industry Organization
Director of the Taiwan Digital Health Industry Development Association
Independent Director of ASUSTEK COMPUTER INC.
Independent Director of Delta Electronics, Inc
Independent Director of Coretronic Corporation
Independent Director
CHEN, RUI-XIN
Key Experience
・
・
・
・
・
・
・
Former Deputy Director of the Trademark Office, Central Standards Bureau, Ministry of Economic Affairs
Former Director of the Legal Affairs Department of Taiwan Intellectual Property Office
Judicial Yuan’s “Theoretical Course for Training High Administrative Court Judges” Lecture
“Patent Law and Trademark Law” Course Lecture at the Academy for the Judiciary
Passed the Financial Personnel Legal Section of the Higher Education Examination
Completion of Master’s Program in Legal Studies at National Chengchi University
Graduated from the Law Department of National Chung Hsing University
Independent Director
LIU, MEI-AN
Key Experience
・
・
・
・
・
・
・
・
Executive Deputy General Manager, Fubon Financial Holdings Venture Capital (Co., Ltd.)
General Manager of Fubon Health Management Consulting (Co., Ltd.)
General Manager of Fuyi Health Management Consulting Co., Ltd.
Deputy General Manager of President Life Sciences Co., Ltd
Executive Vice President of Tongcheng Biosystems
Ph.D. in Cell Biology from Harvard University, USA
Master of Science in Entomology from the University of Massachusetts
Bachelor’s degree, Department of Plant Diseases and Pests, National Taiwan University
Independent Director
LAI, CHUN-LING
Key Experience
・
・
・
・
・
・
・
・
・
・
Chairman of Thuncloud Inc.
Founder and Chairman of Thunder Tiger Corporation
Chairman of TTBIO CORP.
Chairman of THUNDER TIGER MODEL (B.V.I) CO., LTD.
Chairman of Associated Electrics, Inc.
Chairman of Thunder Tiger Europe GmbH
Supervisor
of the National Federation of Industries
Director of Taiwan Aerospace Industry Association
Honorary Chairman of the Chinese Taipei Aerosports Association
Honorary Chairman of Taiwan Toy & Children’s Article Manufacturers Association
Salary and Remuneration Committee
To enhance the company’s policies and systems regarding director and manager compensation, the Salary and Remuneration Committee was established in December 2011. The organizational rules for the committee were formulated to ensure compliance. The fifth Salary and Remuneration Committee, established in 2022, consists of three independent directors. The committee regularly reviews the policies, systems, standards, and structures of director and manager compensation, as well as performance objectives and evaluation methods.
The Salary and Remuneration Committee holds at least two meetings each year. For details on the meetings and member attendance, please refer to the company’s annual reports.
Salary and Remuneration Committee List
Job Title
Name
Independent Director
LIU, MEI-AN (President)
Independent Director
ZENG, HUI-JIN
Independent Director
CHEN, RUI-XIN
Independent Director
LAI, CHUN-LING
Annual Focus and Operations of the Remuneration
Committee
The committee faithfully performs its duties with the care of a good steward and submits its suggestions to the board of directors for discussion.
Annual Focus and Operations of the Remuneration Committee (2024)
Annual Focus and Operations of the Remuneration Committee (2023)
Annual Focus and Operations of the Remuneration Committee (2022)
Audit Committee
The Company established its first Audit Committee on July 5, 2022, composed of all independent directors and governed by the “Audit Committee Organizational Rules.” The main purpose of the Audit Committee is to oversee the following matters:
1. Ensuring proper presentation of the company’s financial statements.
2. Selection (dismissal) of auditors and their independence and performance.
3. Effective implementation of the company’s internal controls.
4. Ensuring the company complies with relevant laws and regulations.
5. Management and control of existing or potential risks of the company.
Committee members shall faithfully perform their duties stipulated in the organizational regulations with the diligence of good managers, be accountable to the Board of Directors, and submit their proposals to the Board of Directors for resolution.
Audit Committee List
Job Title
Name
Independent Director
ZENG, HUI-JIN (Chairman)
Independent Director
LIU, MEI-AN
Independent Director
CHEN, RUI-XIN
Independent Director
LAI, CHUN-LING
Annual Focus and Operations of the Audit Committee
Annual focus and operations of the Remuneration Committee (2024)
Annual focus and operations of the Remuneration Committee (2023)
Annual focus and operations of the Remuneration Committee (2022)
Communication with Independent Directors, the Internal
Audit Supervisor, and the Accountant
Communication with Independent Directors, Internal Audit, and Accountant (2024)
Communication with Independent Directors, Internal Audit, and Accountant (2023)
Communication with Independent Directors, Internal Audit, and Accountant (2022)
Integrity Management Policy

Based on the principles of integrity, transparency, and responsibility, our company has established a policy grounded in honesty. We have also implemented strong corporate governance and risk control mechanisms to create a sustainable business environment for long-term development.
The following relevant management regulations have been established:



The Code of Ethical Conduct outlines preventive measures against the following behaviors:
1. Preventing conflicts of interest.
2. Avoiding opportunities for personal gain.
3. Confidentiality obligations.
4. Fair business practices.
5. Protecting and appropriately using assets.
6. Complying with laws and regulations.
7. Encouraging the reporting of any illegal acts or violations of the Code of Ethical Conduct.
8. Disciplinary measures.
The company reinforces ethical values internally and encourages employees to report any suspected or identified violations of laws, regulations, or the Code of Ethical Conduct to supervisors, managers, internal auditors, or other appropriate personnel. The company has established a concrete whistleblowing system and is committed to fully protecting the safety of employees who report illegal activities, ensuring protection from retaliation.
Safety and Health Policy

Bionime Corporation is a professional manufacturer of blood glucose monitoring systems. Since its establishment, we have deeply understood that employees and supplier partners are the most important assets for the sustainable development of the company. Therefore, during the research and development, manufacturing, testing, and sales processes of our products, in addition to complying with safety and health regulations and other related requirements, we strive for continuous improvement in safety and health measures. This aims to eliminate unsafe behaviors, environments, and equipment, prevent occupational hazards, and fulfill our responsibility to protect the safety and health of our employees. At the same time, we interpret our corporate action goals through Safety, Health, and Environmental (SHE) principles.



Our safety and health policy is as follows
Ensuring the safety and
health of employees
The primary responsibility and duty of all company supervisors.
Providing a safe and
healthy workplace
Prevent work-related injuries, health impairments, illnesses, and accidents to safeguard the safety and health of all employees, as well as suppliers, contractors, and visitors entering the company.
Complying with safety
and health regulations
Complying with national safety and health laws, regulations, and other applicable requirements, and developing relevant standard operating procedures and methods.
Continuously improving
safety and health performance
Continuously improving the safety and health management system and safety and health performance.
Establishing effective
communication channels
Providing consultation and participation channels for workers and their representatives, and encouraging employees to offer suggestions for safety and health. Establishing and maintaining good communication channels between company management and employees. Communicating this policy and addressing safety and health issues to employees, suppliers, customers, contractors, and stakeholders.
Reducing workplace
safety and health risks
Eliminating workplace hazards through elimination, substitution, engineering controls, administrative controls, education and training, and providing appropriate personal protective equipment, to minimize workplace risks.
Bionime Corporation’s Whistleblowing and Complaint Handling System

1. Purpose
To implement the company’s integrity management and employee conduct code, this system is established to provide employees and relevant whistleblowers with a channel to report any illegal activities or violations of the integrity management principles or code of conduct. The system ensures the legal rights and interests of whistleblowers and relevant individuals, helps resolve any unreasonable treatment resulting from violations of social responsibility, and promotes labor-management harmony.
2. Scope and Applicability
2.1
2.2
Scope of Application: This system applies to Bionime Corporation and its subsidiaries.
Applicable Individuals: All employees, suppliers, customers, and other relevant personnel who discover the following situations may submit a report.
2.2.1
2.2.22.2.3
2.2.4
2.2.5
2.2.6
Violations of the company’s integrity management and corporate culture code.
Violations of the company’s employee conduct code.
Workplace legal violations, including but not limited to any form of discrimination, sexual harassment, and other types of harassment.
Violations of the company’s current management regulations, systems, or business operations that harm an individual’s legal rights and interests.
Disclosure of the company’s confidential information, as well as employee or customer information.
Accepting bribes, engaging in favoritism, or colluding in fraudulent activities concerning supervisory or oversight matters, either directly or indirectly seeking unlawful benefits for oneself or others.
3. Responsible Unit
3.1
3.2
3.3
The respective department supervisors and the human resources unit: Responsible for handling reports from internal employees or other stakeholders.
Spokesperson: Responsible for handling reports from shareholders, investors, and other stakeholders.
Audit Department: Responsible for handling reports from the company’s suppliers, contractors, or customers.
4. The reporting format should be in writing as a general principle.
The whistleblower’s name, department, and job title; the name, department, and job title of the person being reported; the date and description of the incident. If the report is made by phone, a written explanation should be provided afterward.
5. Reporting Channels
5.1
5.2
5.3
Email
5.1.1 Human Resources Department:HR@bionime.com
5.1.2 Deputy Spokesperson:investor@bionime.com
Phone Reporting: Stakeholders can find the contact information in the “Stakeholders” section on the company’s Postal
Postal Reporting: Submit a paper report to the company’s registered address, clearly indicating that it should be opened by the internal audit department.
6. Cases Not Accepted for Reporting
6.1
6.2
6.3
6.4
6.5
Anonymous reports or reports made without the whistleblower’s real name, and without providing contact information such as a phone number or mailing address.
Reports that concern matters not included within the scope of the reporting criteria.
Reports that are malicious, false, or clearly inconsistent with facts, logic, or established principles.
The same matter is already under investigation or being handled by other authorities, or has already been reported by someone else. However, if a later report provides crucial evidence that is beneficial to the investigation, it will not be excluded.
The same matter has already been decided not to be accepted for investigation, or has been closed after verification. However, if the whistleblower can present concrete new evidence demonstrating the need for a re-investigation, it will not be excluded.
7. Complaint Handling Process
7.1
7.2
7.3
7.4
Upon receiving a complaint from an employee via the employee email or other grievance systems regarding improper disciplinary actions, the complaint will be reported to the second-level supervisor of the accused and a designated personnel will be assigned to handle the matter. If the complaint is related to issues with a supervisor’s management, it will be handled impartially by the Personnel Evaluation Committee, and the results of the investigation will be communicated to the complainant.
Upon receiving a report, if the investigation is not completed within thirty working days, the unit handling the report should notify the whistleblower, who has provided contact information, in an appropriate manner.
The investigation unit should prepare an investigation report within thirty working days after completing the investigation. After the report is reviewed and finalized, the results should be communicated to the unit handling the report within fifteen working days. If the investigation confirms the complaint is valid, the findings, with any information that could reveal the whistleblower’s identity removed, should be sent to the Human Resources Department and other relevant units.
The unit handling the report should notify the whistleblower in writing or through another method within fifteen working days after receiving the investigation results.
8. Protective Measures
8.1
8.2
8.3
The whistleblower’s identity and personal information must be kept confidential and should not be disclosed in a manner that could reveal their identity.
The whistleblower shall not be dismissed, demoted, reassigned, have their salary reduced, or have any legally or contractually entitled benefits impaired, nor be subject to any other unfavorable treatment as a result of reporting the case.
The unit handling the report, the investigation unit, and personnel involved in the investigation or cooperating with the investigation shall not face improper treatment as a result of their involvement in accepting, investigating, or assisting with the whistleblowing case.
9. Records and Retention
9.1
9.2
The unit handling the report and the investigation unit should maintain complete records of all relevant information.
The report handling, investigation process, and investigation results should be retained in written or electronic form and stored for at least five years. If a lawsuit related to the whistleblowing content occurs before the retention period expires, the records must be kept until the lawsuit is concluded.
Information Security Risk Management Framework

Information Security Risk Assessment Analysis and Response Measures:
Our company refers to the COSO framework to assess and measure elements such as the control environment, risk assessment, control activities, information and communication, and monitoring, in order to establish an operational management mechanism for the enterprise. Based on the “Guidelines for Establishing Internal Control Systems for Publicly Listed Companies,” we broadly define the functions of risk management and internal control. The following outlines the information security risk management mechanism:
1. Information Security Risk Management Framework
1.
2.
3.
Our company has established a cross-departmental Information Security Committee, with the General Manager serving as the Chief Convenor, the Director of R&D acting as the Management Representative, and concurrently holding the positions of Chief Information Security Officer and Chief Personal Data Protection Officer.
We have established information security management objectives and policies, as well as personal data management policies, and regularly review and revise them.
We regularly hold management review meetings to assess the implementation of the information security management system and the personal data management system.
2. Information Security Control Measures
1.
2.
3.
4.
5.
6.
7.
8.
9.
Establish regular inventories of information assets and personal data, conduct risk management based on information security and personal data risk assessments, and implement various control measures.
Annually conduct information security and personal data protection training and awareness programs. All new employees are required to sign an information security confidentiality agreement.
Outsourced vendors must sign a confidentiality agreement to ensure that those using the information services provided by our company or performing related information tasks have the responsibility and obligation to protect the company’s information assets obtained or used, preventing unauthorized access, alteration, destruction, or improper disclosure.
Critical information systems or equipment have established appropriate backup, redundancy, or monitoring mechanisms, and regular drills are conducted to maintain their availability.
All personal computers are equipped with antivirus software, and the virus definitions are regularly updated. The use of unauthorized software is strictly prohibited.
Employees are required to properly manage and use their accounts, passwords, and permissions, and to change their passwords regularly.
We have established standard procedures for responding to and reporting information security incidents. The Information Security Emergency Response Team is responsible for handling information security incidents to ensure timely action and prevent the escalation of damage.
A business continuity management mechanism has been established, with regular testing and drills conducted to maintain its applicability.
Internal audits are conducted regularly every year to ensure the effectiveness of the information security and personal data protection management systems.
3. Information Security Risk Management and Review Implementation
1.
2.
3.
4.
Our company has passed third-party audit verification for ISO 27001 (Information Security Management System) and ISO 27701 (Personal Information Management System).
Through the implementation of ISO 27001 (Information Security Management System), we strengthen our ability to respond to information security incidents and protect the security of the company’s and clients’ information assets.
Through the implementation of ISO 27701 (Personal Information Management System), we assess the risks associated with the personal data held by the company, comply with the personal data protection regulations of various countries, and ensure the security of both the company’s and clients’ personal data.
The information security and personal data protection inspection and control operations shall be listed as annual audit items. The auditing unit shall conduct at least one audit per year. Additionally, the company shall conduct self-inspections based on the internal control system annually, summarize the effectiveness of internal control implementation, and report the findings to the Board of Directors for review and confirmation. Based on the evaluation results, an internal control system statement shall be issued.
Bionime Corporation │ Customer Service Hotline 0800-371-688 │ Service Hours: Monday to Friday 8:30-22:00

Company Profile
Company Name
Bionime Corporation
Abbreviation
Bionime
Stock Code
4737
Market Type
Listed Company
Industry Type
Biotechnology and Medical Care
Business
Manufacturing
Selling Medical Instrument
Biotechnology Service
Examine In Pharmaceuticals
Selling Precision Instruments
Date of Establishment
2003/4/14
Date of Listing
2010/12/23
Capital
NT$ 610,387,940
Chairman
Roy Huang
Principal Office
No. 100, Sec. 2 Daqing St., South Dist., Taichung City 40242, Taiwan (R.O.C.)
Stock Transfer Agent
Chinatrust Commercial Bank
Certified Public Accountant
KPMG
Tel
886 4 2369 2388
Fax
886 4 2261 7586
info@bionime.com
Website
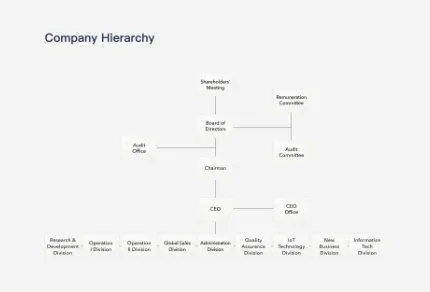
Division Functions
Division
Functions
Remuneration Committee
Assist the board of directors to implement and evaluate the company’s overall remuneration and welfare policies, and the remuneration of directors, supervisors, and managers.
Audit Committee
The Audit Committee assists the board in fulfilling its oversight of the quality and integrity of the accounting, auditing, reporting, and financial control practices of the company.
Audit Office
Report to the Board and Administration Division to take in the extent to which the operational effectiveness and efficiency objectives have been achieved as well as the reliability of the financial statements and relevant laws and regulations to comply with the relevant internal controls. Point out internal control deficiencies and any suggestions as well as abnormal matters tracking improvement.
CEO Office
Carry out all company affairs in accordance with the resolutions of the shareholders’ meeting and the Board of Directors, as well as the instructions of the Board.
Research & Development Division
Responsible for the biochemical development of blood glucose test strips, new products, clinical certification, and matters related to intellectual property and patents.
Operation Division
Oversee the company’s production, outsourcing, logistics management, mold development, and automation matters to achieve the company’s annual production plans and goals.
Sales Division
Oversee the company’s product sales, customer service, market development planning, new product development plans, IoT product service planning and design, as well as product and brand marketing communication planning and execution.
Administration Division
Oversee the company’s financial management, accounting, human resources, and administrative affairs.
Quality Assurance Division
Responsible for the company’s quality system, quality monitoring and improvement, and the validation of new product development.
IOT Technology Division
Responsible for the software research and development, operation and maintenance of the IoT cloud platform, POCT system for remote care, as well as platform system integration and technical support.
New Business Division
Responsible for the electromechanical development and integration of products, as well as the research and evaluation of innovative product development.
Information Tech Division
Responsible for the overall information systems of the company, as well as the planning, execution, and maintenance of information integration and automation for smart manufacturing.
Internal Audit

Our company’s Audit Department directly reports to the Board of Directors and is headed by an audit supervisor. The audit unit carries out regular audits according to the annual internal audit plan and conducts ad-hoc project audits as needed. The audit department also guides each department in performing self-assessment of the internal control system and reports the results of these self-assessments, along with the execution of audits, to the relevant regulatory authorities within the specified timeframe.
Stakeholders

Supplier Area
To sustainably promote the development of Bionime’s business, it is essential to establish partnerships with suppliers across the supply chain. All partners are expected to comply with the social responsibility standards of the supply chain. Our company conducts EHS (Environmental, Health, and Safety) advocacy and audits with key suppliers, focusing on the implementation of health checks for employees, prevention of occupational hazards, fire safety, environmental hygiene, and other related areas. Suppliers are expected to fully cooperate with these requirements and make necessary improvements to ensure the fulfillment of corporate social responsibility.
Key Issues



Communication Channels, Response Methods, and Communication Frequency
Established a Secure Supply Chain Partner Management Policy, with regular two-way communication with suppliers.
Supplier Contact Point
Logistics Management Department
Manager:Joanne Chang
E-mail:joanne.chang@bionime.com
Consumer Area
Bionime Corporation, established in 2003, is currently focused on developing “Self-Blood Glucose Monitoring Systems” tailored for diabetes patients and professional healthcare institutions. By successfully integrating cutting-edge technologies in healthcare, chemistry, electronics, and precision manufacturing, Bionime has developed unique patented technologies that deliver highly accurate and stable blood glucose testing systems.Today, Bionime products are sold in over 80 countries worldwide. Our products have excelled in clinical testing and evaluations by internationally recognized third-party organizations. The unique and superior qualities of our technologies have also been featured in many prestigious international professional journals. Thanks to the outstanding performance of our products and the strong support from customers, Bionime has become a well-known brand in several countries. We are confident in our ability to become the leading brand in home blood glucose monitoring systems.
Key Issues


Communication Channels, Response Methods, and Communication Frequency
Dedicated 0800 hotline for immediate customer inquiries.
Supplier Contact Point
Customer Service Department
Assistant Manager:Zoe Ho
E-mail:info@bionime.com
Toll-Free Hotline:0800-371-688
Employee Area
Bionime offers multiple channels for feedback and has established an employee mailbox, allowing staff to express their opinions freely. Additionally, company-wide operational updates are communicated through email and the internal corporate website.
Key Issues




Communication Channels, Response Methods, and Communication Frequency
Regularly announcing various employee benefits (such as self-improvement activities, travel, etc.), welfare committee information, performance assessments, two-way feedback sessions, training course details, employee suggestion boxes, and diverse communication channels like ‘Meet the CEO’ sessions for new hires.
Supplier Contact Point
Human Resources Department
Manager:Fish Chen
E-mail:hr@ bionime.com
Investor Relations Area
Our company values building strong communication channels with investors. If investors have any questions, they can contact our designated representative.
Key Issues
Company financial statements, business-related, and operational information.
Communication Channels, Response Methods, and Communication Frequency
1.
2.
3.
4.
Irregularly announcing company operational information on the Public Information Observatory and Bionime’s official website.
Holding an annual shareholders’ meeting to update shareholders on the company’s operational status.
Hosting an annual institutional investor conference to brief institutional investors on the company’s operations.
Establishing a spokesperson system for periodic communication with investors.
Investor Contact Window
Acting Spokesperson
Manager:Sherry Huang
E-mail:investor@bionime.com
Board of Directors
The Board of Directors prioritizes the interests of the company and its shareholders. Its members are diverse, with professional backgrounds and industry experience, working together to ensure effective corporate governance.
Director
Roy Huang
Key Experience
.
.
.
Chairman and CEO of Bionime Corporation
General Manager of ECHOWELL ELECTRONIC CO., LTD.
National Taipei Institute of Technology
Multiple Positions Held
.
.
.
.
.
.
.
.
.
Chairman and CEO of Bionime Corporation
Bionime Inc. Legal Chairman
Legal Chairman of Bionime Biotechnology (Shenzhen) Co., Ltd.
Legal Chairman of Bionime Biotechnology (Pingtan) Co., Ltd.
Bionime GmbH Legal Chairman
Bionime USA Corp. Legal Chairman
Bionime Malaysia SDN BHD Director
Bionime Australia Pty Ltd Director
Legal Representative and Director of BONRAYBIO CO., LTD.
Director
LENG, CHUN-SHENG
Key Experience
.
.
Chairman, CEO, and Vice President of Tonghua Dongbao Pharmaceutical Co., Ltd.
Ph.D. in Cell Biology from Liaoning Normal University
Multiple Positions Held
.
.
.
Chairman and CEO of Tonghua Dongbao Pharmaceutical Co., Ltd.
Chairman of Beijing Dongbao Biotechnology Co., Ltd.
Executive Director of Tonghua Tongbo Biopharmaceutical Co., Ltd.
Director
ZHANG, WEN-HAI
Key Experience
.
.
Vice President of Tonghua Dongbao Pharmaceutical Co., Ltd.
TCM Pharmacy Program, Harbin University of Commerce
Multiple Positions Held
.
.
.
Director of Tonghua Dongbao Pharmaceutical Co., Ltd.
Vice General Manager of Tonghua Dongbao Pharmaceutical Co., Ltd.
Chairman of Shanghai Juyi Network Technology Co., Ltd.
Director
LIOU, XIU-MEI
Key Experience
.
.
Vice General Manager of HUA ENG WIRE AND CABLE CO., LTD.
Master of Accounting, Long Island University, USA
Multiple Positions Held
.
.
.
.
.
.
.
Legal Representative and Director of Bionime Corporation
Legal Representative and Director of Xiamen Ecotek PRC CO., LTD.
Legal Representative and Director of Wafer Works Corporation
Legal Representative and Director of Asia Pacific Telecom Co., Ltd.
Legal Representative and Director of CO-TECH DEVELOPMENT CORP.
Supervisor of HUA HO ENGINEERING CO., LTD.
Manager of HUA ENG WIRE AND CABLE CO., LTD.
Director
Yanben Investment
Co., Ltd.
Key Experience
.
.
Legal Director of Advantech Co., Ltd.
Legal Representative and Director of Chuanian IoT Investment Co., Ltd.
Independent Director
ZENG, HUI-JIN
Key Experience
.
.
.
Partner, Deputy Managing Director, Chief Operating Officer of the Audit Department, and Chief Strategy Officer of PwC Taiwan
National Taiwan University/Fudan University School of Management Overseas Master’s Degree
Master of Accounting, National Chengchi University
Multiple Positions Held
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Advisory Member of the Bio Taiwan Committee.
Advisor of the Biomedical Translation Research Center.
T-E Pharma Holding (Cayman) Director
Onward Therapeutics SA (Switzerland) Independent Director
HanchorBio Inc. (Cayman) Director
Legal Representative and Director of Bonraybio Corporation
Legal Representative and Director of AP Biosciences
Director of BRIM Biotechnology, Inc.
Director of New Taipei City Contemporary Legend Cultural and Arts Foundation
Director of the Chi Po-lin Foundation.
Supervisor of the Medical and Pharmaceutical Industry Technology and Development Center.
Supervisor of the Food Industry Research and Development Institute
Vice Chairman of the A Community Of Minds Pushing Forward Advancements In Precision Medicine.
Executive Supervisor of the Taiwan Bio Industry Organization
Director of the Taiwan Digital Health Industry Development Association
Independent Director of ASUSTEK COMPUTER INC.
Independent Director of Delta Electronics, Inc
Independent Director of Coretronic Corporation
Independent Director
CHEN, RUI-XIN
Key Experience
.
.
.
.
.
.
.
Former Deputy Director of the Trademark Office, Central Standards Bureau, Ministry of Economic Affairs
Former Director of the Legal Affairs Department of Taiwan Intellectual Property Office
Judicial Yuan’s “Theoretical Course for Training High Administrative Court Judges” Lecture
“Patent Law and Trademark Law” Course Lecture at the Academy for the Judiciary
Passed the Financial Personnel Legal Section of the Higher Education Examination
Completion of Master’s Program in Legal Studies at National Chengchi University
Graduated from the Law Department of National Chung Hsing University
Independent Director
LIU, MEI-AN
Key Experience
.
.
.
.
.
.
.
.
Executive Deputy General Manager, Fubon Financial Holdings Venture Capital (Co., Ltd.)
General Manager of Fubon Health Management Consulting (Co., Ltd.)
General Manager of Fuyi Health Management Consulting Co., Ltd.
Deputy General Manager of President Life Sciences Co., Ltd
Executive Vice President of Tongcheng Biosystems
Ph.D. in Cell Biology from Harvard University, USA
Master of Science in Entomology from the University of Massachusetts
Bachelor’s degree, Department of Plant Diseases and Pests, National Taiwan University
Independent Director
LAI, CHUN-LING
Key Experience
.
.
.
.
.
.
.
.
.
.
Chairman of Thuncloud Inc.
Founder and Chairman of Thunder Tiger Corporation
Chairman of TTBIO CORP.
Chairman of THUNDER TIGER MODEL (B.V.I) CO., LTD.
Chairman of Associated Electrics, Inc.
Chairman of Thunder Tiger Europe GmbH
Supervisor
of the National Federation of Industries
Director of Taiwan Aerospace Industry Association
Honorary Chairman of the Chinese Taipei Aerosports Association
Honorary Chairman of Taiwan Toy & Children’s Article Manufacturers Association
Salary and Remuneration Committee
To enhance the company’s policies and systems regarding director and manager compensation, the Salary and Remuneration Committee was established in December 2011. The organizational rules for the committee were formulated to ensure compliance. The fifth Salary and Remuneration Committee, established in 2022, consists of three independent directors. The committee regularly reviews the policies, systems, standards, and structures of director and manager compensation, as well as performance objectives and evaluation methods.
The Salary and Remuneration Committee holds at least two meetings each year. For details on the meetings and member attendance, please refer to the company’s annual reports.
Salary and Remuneration Committee List
Job Title
Name
Independent Director
LIU, MEI-AN (President)
Independent Director
ZENG, HUI-JIN
Independent Director
CHEN, RUI-XIN
Independent Director
LAI, CHUN-LING
Audit Committee
The Company established its first Audit Committee on July 5, 2022, composed of all independent directors and governed by the “Audit Committee Organizational Rules.” The main purpose of the Audit Committee is to oversee the following matters:
1.
2.
3.
4.
5.
Ensuring proper presentation of the company’s financial statements.
Selection (dismissal) of auditors and their independence and performance.
Effective implementation of the company’s internal controls.
Ensuring the company complies with relevant laws and regulations.
Management and control of existing or potential risks of the company.
Committee members shall faithfully perform their duties stipulated in the organizational regulations with the diligence of good managers, be accountable to the Board of Directors, and submit their proposals to the Board of Directors for resolution.
Audit Committee List
Job Title
Name
Independent Director
ZENG, HUI-JIN (President)
Independent Director
LIU, MEI-AN
Independent Director
CHEN, RUI-XIN
Independent Director
LAI, CHUN-LING
Integrity Management Policy

Based on the principles of integrity, transparency, and responsibility, our company has established a policy grounded in honesty. We have also implemented strong corporate governance and risk control mechanisms to create a sustainable business environment for long-term development.
The following relevant management regulations have been established:



The Code of Ethical Conduct outlines preventive measures against the following behaviors:
1.
2.
3.
4.
5.
6.
7.
8.
Preventing conflicts of interest.
Avoiding opportunities for personal gain.
Confidentiality obligations.
Fair business practices.
Protecting and appropriately using assets.
Complying with laws and regulations.
Encouraging the reporting of any illegal acts or violations of the Code of Ethical Conduct.
Disciplinary measures.
The company reinforces ethical values internally and encourages employees to report any suspected or identified violations of laws, regulations, or the Code of Ethical Conduct to supervisors, managers, internal auditors, or other appropriate personnel. The company has established a concrete whistleblowing system and is committed to fully protecting the safety of employees who report illegal activities, ensuring protection from retaliation.
Safety and Health Policy

Bionime Corporation is a professional manufacturer of blood glucose monitoring systems. Since its establishment, we have deeply understood that employees and supplier partners are the most important assets for the sustainable development of the company. Therefore, during the research and development, manufacturing, testing, and sales processes of our products, in addition to complying with safety and health regulations and other related requirements, we strive for continuous improvement in safety and health measures. This aims to eliminate unsafe behaviors, environments, and equipment, prevent occupational hazards, and fulfill our responsibility to protect the safety and health of our employees. At the same time, we interpret our corporate action goals through Safety, Health, and Environmental (SHE) principles.



Our safety and health policy is as follows
Ensuring the safety and health of employees
The primary responsibility and duty of all company supervisors.
Providing a safe and healthy workplace
Prevent work-related injuries, health impairments, illnesses, and accidents to safeguard the safety and health of all employees, as well as suppliers, contractors, and visitors entering the company.
Complying with safety and health regulations
Complying with national safety and health laws, regulations, and other applicable requirements, and developing relevant standard operating procedures and methods.
Continuously improving safety and health performance
Continuously improving the safety and health management system and safety and health performance.
Establishing effective communication channels
Providing consultation and participation channels for workers and their representatives, and encouraging employees to offer suggestions for safety and health. Establishing and maintaining good communication channels between company management and employees. Communicating this policy and addressing safety and health issues to employees, suppliers, customers, contractors, and stakeholders.
Reducing workplace safety and health risks
Eliminating workplace hazards through elimination, substitution, engineering controls, administrative controls, education and training, and providing appropriate personal protective equipment, to minimize workplace risks.
Bionime Corporation’s Whistleblowing and Complaint Handling System

1. Purpose
To implement the company’s integrity management and employee conduct code, this system is established to provide employees and relevant whistleblowers with a channel to report any illegal activities or violations of the integrity management principles or code of conduct. The system ensures the legal rights and interests of whistleblowers and relevant individuals, helps resolve any unreasonable treatment resulting from violations of social responsibility, and promotes labor-management harmony.
2. Scope and Applicability
2.1
2.2
Scope of Application: This system applies to Bionime Corporation and its subsidiaries.
Applicable Individuals: All employees, suppliers, customers, and other relevant personnel who discover the following situations may submit a report.
2.2.1
2.2.2
2.2.3
2.2.4
2.2.5
2.2.6
Violations of the company’s integrity management and corporate culture code.
Violations of the company’s employee conduct code.
Workplace legal violations, including but not limited to any form of discrimination, sexual harassment, and other types of harassment.
Violations of the company’s current management regulations, systems, or business operations that harm an individual’s legal rights and interests.
Disclosure of the company’s confidential information, as well as employee or customer information.
Accepting bribes, engaging in favoritism, or colluding in fraudulent activities concerning supervisory or oversight matters, either directly or indirectly seeking unlawful benefits for oneself or others.
3. Responsible Unit
3.1
3.2
3.3
The respective department supervisors and the human resources unit: Responsible for handling reports from internal employees or other stakeholders.
Spokesperson: Responsible for handling reports from shareholders, investors, and other stakeholders.
Audit Department: Responsible for handling reports from the company’s suppliers, contractors, or customers.
4. The reporting format should be in writing as a general principle.
The whistleblower’s name, department, and job title; the name, department, and job title of the person being reported; the date and description of the incident. If the report is made by phone, a written explanation should be provided afterward.
5. Reporting Channels
5.1
5.1.1
5.1.2
Human Resources Department:HR@bionime.com
Deputy Spokesperson:investor@bionime.com
5.2
5.3
Phone Reporting: Stakeholders can find the contact information in the “Stakeholders” section on the company’s Postal
Postal Reporting: Submit a paper report to the company’s registered address, clearly indicating that it should be opened by the internal audit department.
6. Cases Not Accepted for Reporting
6.1
6.2
6.3
6.4
6.5
Anonymous reports or reports made without the whistleblower’s real name, and without providing contact information such as a phone number or mailing address.
Reports that concern matters not included within the scope of the reporting criteria.
Reports that are malicious, false, or clearly inconsistent with facts, logic, or established principles.
The same matter is already under investigation or being handled by other authorities, or has already been reported by someone else. However, if a later report provides crucial evidence that is beneficial to the investigation, it will not be excluded.
The same matter has already been decided not to be accepted for investigation, or has been closed after verification. However, if the whistleblower can present concrete new evidence demonstrating the need for a re-investigation, it will
not be excluded.
7. Complaint Handling Process
7.1
7.2
7.3
7.4
Upon receiving a complaint from an employee via the employee email or other grievance systems regarding improper disciplinary actions, the complaint will be reported to the second-level supervisor of the accused and a designated personnel will be assigned to handle the matter. If the complaint is related to issues with a supervisor’s management, it will be handled impartially by the Personnel Evaluation Committee, and the results of the investigation will be communicated to the complainant.
Upon receiving a report, if the investigation is not completed within thirty working days, the unit handling the report should notify the whistleblower, who has provided contact information, in an appropriate manner.
The investigation unit should prepare an investigation report within thirty working days after completing the investigation. After the report is reviewed and finalized, the results should be communicated to the unit handling the report within fifteen working days. If the investigation confirms the complaint is valid, the findings, with any information that could reveal the whistleblower’s identity removed, should be sent to the Human Resources Department and other relevant units.
The unit handling the report should notify the whistleblower in writing or through another method within fifteen working days after receiving the investigation results.
8. Protective Measures
8.1
8.2
8.3
The whistleblower’s identity and personal information must be kept confidential and should not be disclosed in a manner that could reveal their identity.
The whistleblower shall not be dismissed, demoted, reassigned, have their salary reduced, or have any legally or contractually entitled benefits impaired, nor be subject to any other unfavorable treatment as a result of reporting the case.
The unit handling the report, the investigation unit, and personnel involved in the investigation or cooperating with the investigation shall not face improper treatment as a result of their involvement in accepting, investigating, or assisting with the whistleblowing case.
9. Records and Retention
9.1
9.2
The unit handling the report and the investigation unit should maintain complete records of all relevant information.
The report handling, investigation process, and investigation results should be retained in written or electronic form and stored for at least five years. If a lawsuit related to the whistleblowing content occurs before the retention period expires, the records must be kept until the lawsuit is concluded.
Information Security Risk Management Framework

Information Security Risk Assessment Analysis and Response Measures:
Our company refers to the COSO framework to assess and measure elements such as the control environment, risk assessment, control activities, information and communication, and monitoring, in order to establish an operational management mechanism for the enterprise. Based on the “Guidelines for Establishing Internal Control Systems for Publicly Listed Companies,” we broadly define the functions of risk management and internal control. The following outlines the information security risk management mechanism:
1. Information Security Risk Management Framework
1.
2.
3.
Our company has established a cross-departmental Information Security Committee, with the General Manager serving as the Chief Convenor, the Director of R&D acting as the Management Representative, and concurrently holding the positions of Chief Information Security Officer and Chief Personal Data Protection Officer.
We have established information security management objectives and policies, as well as personal data management policies, and regularly review and revise them.
We regularly hold management review meetings to assess the implementation of the information security management system and the personal data management system.
2. Information Security Control Measures
1.
2.
3.
4.
5.
6.
7.
8.
9.
Establish regular inventories of information assets and personal data, conduct risk management based on information security and personal data risk assessments, and implement various control measures.
Annually conduct information security and personal data protection training and awareness programs. All new employees are required to sign an information security confidentiality agreement.
Outsourced vendors must sign a confidentiality agreement to ensure that those using the information services provided by our company or performing related information tasks have the responsibility and obligation to protect the company’s information assets obtained or used, preventing unauthorized access, alteration, destruction, or improper disclosure.
Critical information systems or equipment have established appropriate backup, redundancy, or monitoring mechanisms, and regular drills are conducted to maintain their availability.
All personal computers are equipped with antivirus software, and the virus definitions are regularly updated. The use of unauthorized software is strictly prohibited.
Employees are required to properly manage and use their accounts, passwords, and permissions, and to change their passwords regularly.
We have established standard procedures for responding to and reporting information security incidents. The Information Security Emergency Response Team is responsible for handling information security incidents to ensure timely action and prevent the escalation of damage.
A business continuity management mechanism has been established, with regular testing and drills conducted to maintain its applicability.
Internal audits are conducted regularly every year to ensure the effectiveness of the information security and personal data protection management systems.
3. Information Security Risk Management and Review Implementation
1.
2.
3.
4.
Our company has passed third-party audit verification for ISO 27001 (Information Security Management System) and ISO 27701 (Personal Information Management System).
Through the implementation of ISO 27001 (Information Security Management System), we strengthen our ability to respond to information security incidents and protect the security of the company’s and clients’information assets.
Through the implementation of ISO 27701 (Personal Information Management System), we assess the risks associated with the personal data held by the company, comply with the personal data protection regulations of various countries, and ensure the security of both the company’s and clients’ personal data.
The information security and personal data protection inspection and control operations shall be listed as annual audit items. The auditing unit shall conduct at least one audit per year. Additionally, the company shall conduct self-inspections based on the internal control system annually, summarize the effectiveness of internal control implementation, and report the findings to the Board of Directors for review and confirmation. Based on the evaluation results, an internal control system statement shall be issued.